Abstract
Background: Myeloablative conditioning can be given safely to older patients by simply administering busulfan over a longer period (fractionated busulfan regimen) than our "standard" four-day fludarabine-busulfan (Flu-Bu) regimen. (Popat et al Lancet Haematology 2018). To further improve outcomes of this fractionated (f-Bu) regimen in patients undergoing matched donor hematopoietic cell transplantation (HCT), we added cladribine (Clad) to f-Bu-Flu regimen in a prospective clinical trial (NCT02250937). GVHD prophylaxis was tacrolimus and methotrexate (Tac/MTX).
After enrolling the first 29 patients, the study was amended and GVHD prophylaxis was changed to post-transplant cyclophosphamide (PTCy) and tacrolimus for the next 53 patients based on very promising data observed in patients undergoing haploidentical transplantation. We hypothesized that PTCy will reduce GVHD and non-relapse mortality (NRM), thus improving survival in patients undergoing HCT from a matched donor. In this study, we compared these two sequential cohorts with reference to GVHD prophylaxis.
Methods: Between 2/2015 and 12/2018, 82 patients with AML or MDS, 18-70 years of age, with adequate organ function and 8/8-HLA matched related (n=38%) or unrelated (62%) donors were enrolled. The conditioning regimen was f-Bu to target an area under the concentration vs time curve (AUC) of 20,000 ± 12% μmol.min given over a period of 2-3 weeks. The first two doses of busulfan (80 mg/m2 IV each) were administered either consecutively (days -13 and -12) or with further fractionation, one week apart (days -20 and -13) on outpatient basis. Then, inpatient fludarabine 10 mg/m 2, and cladribine 10 mg/m 2 were given followed by Bu on days -6 to -3. GVHD prophylaxis was Tac/Mtx (n=29) or PTCy 50mg/kg on days 3 and 4 followed by tacrolimus from day 5 (n=53).
Results: Baseline characteristics were similar between the PTCy and Tac/Mtx cohorts. The median age was 59 (range, 18-70) and 61 (range, 24-70) years (P=0.20); 49% and 59% had primary induction failure at HCT (P=0.58); High or very-high disease risk index was present in 40% and 41%, (P=0.40); Comorbidity index score >3 was present in 42% and 40% (P=0.24); Donor was a sibling in 34% and 45% (P=0.35), and peripheral blood graft was used in 81% and 76% (P=0.58), respectively in PTCy and Tac/Mtx cohorts.
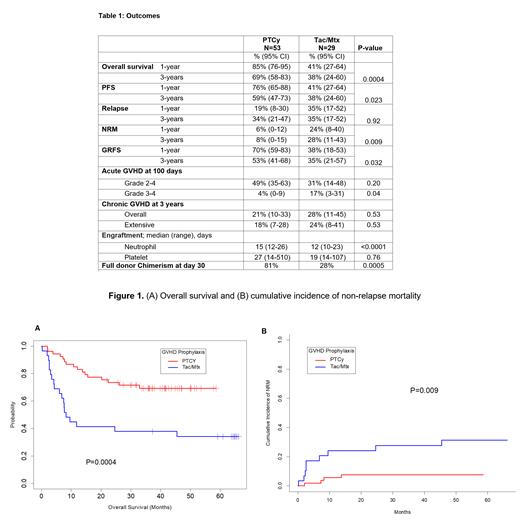
Median follow up was 42.7 months. In the PTCy and Tac/Mtx cohorts, at 3-years overall survival was 69% vs 38% (P=0.0004), NRM was 8% vs 28% (P=0.009) [Table 1, Figure 1], incidence of grade 3-4 acute GVHD at day 100 was 4% vs 17% (P=0.04), and chronic GVHD was 21% vs 28% at 3 years (P=0.53). Median time to neutrophil engraftment was prolonged by 3 days with PTCy (15 vs 12 days; P<0.0001). Full donor chimerism at day 30 was noted in 81% vs 28%, in the PTCy and Tac/Mtx cohorts respectively, (P=0.005). The toxicity profile was similar except neutropenic fever (likely cytokine-related) was higher in PTCy group (60% v/s 24%, P=0.002). Likewise, the incidence of hemorrhagic cystitis was higher in the PTCy group (36% vs 14%, p-0.04). Most of the later events were grade 1 or 2.
Conclusion: Compared with Tac/Mtx, PTCy reduced severe acute GVHD and NRM, and improved survival in AML/MDS patients up to the age of 70 years who received myeloablative fractionated busulfan conditioning and transplant from a matched donor.
Popat: Bayer: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Mehta: CSLBehring: Research Funding; Kadmon: Research Funding; Syndax: Research Funding; Incyte: Research Funding. Hosing: Nkarta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Rezvani: Navan Technologies: Other: Scientific Advisory Board; Caribou: Other: Scientific Advisory Board; GemoAb: Other: Scientific Advisory Board ; GSK: Other: Scientific Advisory Board ; Pharmacyclics: Other: Educational grant, Research Funding; Bayer: Other: Scientific Advisory Board ; Takeda: Other: License agreement and research agreement, Patents & Royalties; Affimed: Other: License agreement and research agreement; education grant, Patents & Royalties, Research Funding; Virogin: Other: Scientific Advisory Board ; AvengeBio: Other: Scientific Advisory Board . Qazilbash: Janssen: Research Funding; Biolline: Research Funding; Oncopeptides: Other: Advisory Board; NexImmune: Research Funding; Angiocrine: Research Funding; Amgen: Research Funding; Bristol-Myers Squibb: Other: Advisory Board. Kadia: Liberum: Consultancy; AstraZeneca: Other; Sanofi-Aventis: Consultancy; Genfleet: Other; Pulmotech: Other; Dalichi Sankyo: Consultancy; Genentech: Consultancy, Other: Grant/research support; Amgen: Other: Grant/research support; Astellas: Other; Jazz: Consultancy; Aglos: Consultancy; Ascentage: Other; Cellonkos: Other; Novartis: Consultancy; Pfizer: Consultancy, Other; AbbVie: Consultancy, Other: Grant/research support; BMS: Other: Grant/research support; Cure: Speakers Bureau. Kantarjian: BMS: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria; Novartis: Honoraria, Research Funding; Jazz: Research Funding; Precision Biosciences: Honoraria; Amgen: Honoraria, Research Funding; Aptitude Health: Honoraria; Immunogen: Research Funding; Ascentage: Research Funding; AbbVie: Honoraria, Research Funding; NOVA Research: Honoraria; Ipsen Pharmaceuticals: Honoraria; Astellas Health: Honoraria; Astra Zeneca: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Shpall: Adaptimmune: Consultancy; Magenta: Consultancy; Novartis: Honoraria; Affimed: Patents & Royalties; Navan: Consultancy; Magenta: Honoraria; Novartis: Consultancy; Takeda: Patents & Royalties; Bayer HealthCare Pharmaceuticals: Honoraria; Axio: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal